Understanding the nature of chemical bonds is crucial for students, researchers, and anyone interested in chemistry. One common question that arises is whether hydrochloric acid (HCl) is ionic or covalent. This question is not only fundamental to chemistry but also has practical applications in various fields, including medicine, engineering, and environmental science. In this article, we will explore the nature of HCl bonds in detail, providing a clear and comprehensive answer. Hydrochloric acid (HCl) is a fascinating compound that plays a significant role in chemical reactions and industrial processes. Its bonding nature has been a topic of discussion among chemists for years, and understanding whether it is ionic or covalent can help clarify its behavior in different environments.
Chemical bonds are the forces that hold atoms together in molecules and compounds. They are broadly classified into two main types: ionic and covalent. While ionic bonds involve the transfer of electrons between atoms, covalent bonds involve the sharing of electrons. Determining the type of bond in a compound like HCl requires a deeper understanding of its molecular structure and the electronegativity difference between its atoms. This article will guide you through the key factors that define HCl's bonding nature.
Hydrochloric acid is widely used in laboratories and industries, making it a critical compound to study. Whether you are a student preparing for exams, a professional working in the chemical industry, or simply curious about chemistry, this article will provide you with valuable insights. By the end of this guide, you will have a clear understanding of whether HCl is ionic or covalent and why it matters. Let’s dive into the details!
Read also:Who Is The Current Owner Of Gucci Discover The Story Behind The Iconic Brand
Table of Contents
Introduction to HCl
Hydrochloric acid (HCl) is a colorless, highly corrosive chemical compound that is commonly used in laboratories and industrial processes. It is a strong acid, meaning it dissociates completely in water to form hydrogen ions (H⁺) and chloride ions (Cl⁻). This dissociation makes HCl an essential reagent in various chemical reactions, including pH regulation, metal cleaning, and the production of organic compounds.
HCl is naturally present in the human stomach, where it aids in digestion by breaking down food particles. Its industrial applications include the production of PVC plastic, the purification of table salt, and the treatment of wastewater. Despite its widespread use, understanding the nature of HCl's chemical bonds is critical to predicting its behavior in different environments.
What Are Ionic Bonds?
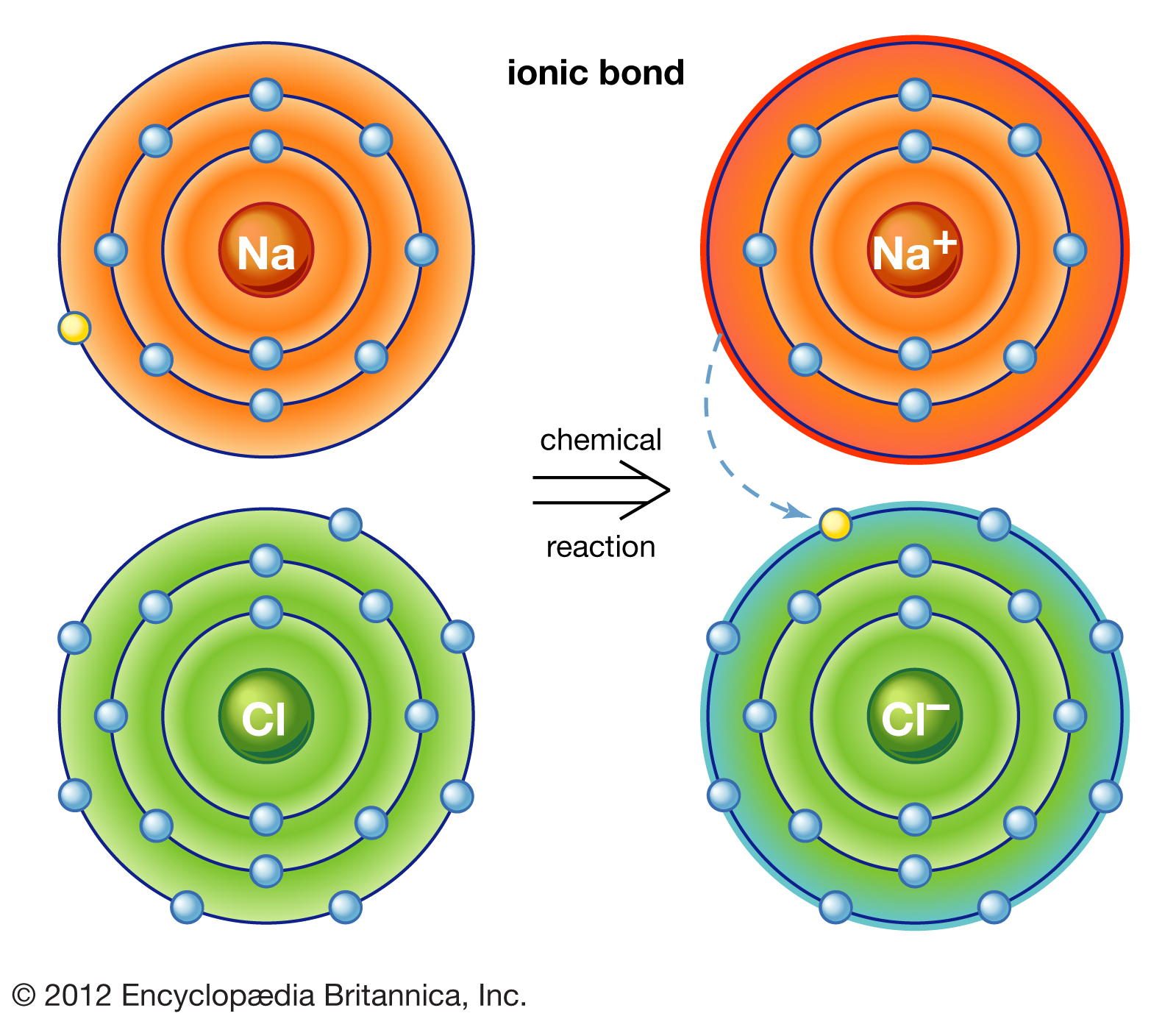
Ionic bonds are a type of chemical bond that occurs when one atom transfers one or more electrons to another atom. This transfer results in the formation of positively charged ions (cations) and negatively charged ions (anions). The electrostatic attraction between these oppositely charged ions holds the compound together.
Examples of compounds with ionic bonds include sodium chloride (NaCl), magnesium oxide (MgO), and calcium carbonate (CaCO₃). Ionic compounds typically have high melting and boiling points, are solid at room temperature, and conduct electricity when dissolved in water or melted. However, HCl does not exhibit these characteristics, which raises questions about its bonding nature.
Characteristics of Ionic Bonds
- Involve the complete transfer of electrons.
- Form between metals and nonmetals.
- Result in crystalline solids with high melting points.
- Conduct electricity in aqueous solutions.
What Are Covalent Bonds?
Covalent bonds, on the other hand, involve the sharing of electrons between atoms. This type of bond typically occurs between nonmetals and is characterized by the formation of molecules. Covalent bonds can be polar or nonpolar, depending on the electronegativity difference between the bonded atoms.
Examples of covalent compounds include water (H₂O), methane (CH₄), and carbon dioxide (CO₂). These compounds generally have lower melting and boiling points compared to ionic compounds and do not conduct electricity in their pure form. HCl shares many characteristics with covalent compounds, which suggests that it may have a covalent bond.
Read also:Dirty Dancing Cast Updates Where Are They Now
Types of Covalent Bonds
- Polar Covalent Bonds: Unequal sharing of electrons due to a significant electronegativity difference.
- Nonpolar Covalent Bonds: Equal sharing of electrons between atoms of similar electronegativity.
Electronegativity and Bond Type
Electronegativity is a measure of an atom's ability to attract electrons in a chemical bond. The difference in electronegativity between two atoms determines the type of bond they form. A large electronegativity difference (greater than 1.7) typically results in an ionic bond, while a smaller difference (less than 1.7) leads to a covalent bond.
In the case of HCl, hydrogen has an electronegativity of 2.20, while chlorine has an electronegativity of 3.16. The difference between these values is 0.96, which falls within the range for a polar covalent bond. This indicates that HCl is not ionic but rather a polar covalent compound.
Molecular Structure of HCl
The molecular structure of HCl consists of one hydrogen atom covalently bonded to one chlorine atom. The bond is polar because chlorine is more electronegative than hydrogen, causing the shared electrons to be pulled closer to the chlorine atom. This creates a partial negative charge on chlorine and a partial positive charge on hydrogen.
The polarity of the HCl bond gives the molecule a dipole moment, making it a polar molecule. This polarity influences HCl's physical and chemical properties, such as its solubility in water and its ability to conduct electricity when dissolved.
Key Features of HCl's Molecular Structure
- Linear shape with a single covalent bond.
- Polar due to electronegativity difference.
- Forms a dipole moment due to uneven electron distribution.
Is HCl Ionic or Covalent?
Based on the analysis of electronegativity and molecular structure, HCl is a polar covalent compound. The bond between hydrogen and chlorine involves the sharing of electrons, but the electrons are not shared equally due to the difference in electronegativity. This unequal sharing results in a polar covalent bond, which is distinct from an ionic bond.
While HCl dissociates into ions (H⁺ and Cl⁻) when dissolved in water, this does not mean it is an ionic compound. The dissociation occurs because of the polar nature of the HCl molecule, which allows it to interact with water molecules. In its pure form, HCl exists as a covalent molecule.
Why HCl is Not Ionic
- Electronegativity difference is less than 1.7.
- Does not form a crystalline solid at room temperature.
- Does not conduct electricity in its pure form.
Properties of HCl
HCl exhibits several unique properties that are influenced by its polar covalent nature. Understanding these properties can provide further insights into its behavior in different environments.
Physical Properties
- Colorless gas at room temperature.
- Highly soluble in water, forming hydrochloric acid.
- Has a pungent odor.
Chemical Properties
- Strong acid that dissociates completely in water.
- Reacts with metals to produce hydrogen gas.
- Used in neutralization reactions with bases.
Applications of HCl
Hydrochloric acid is widely used in various industries due to its strong acidic properties and ability to dissolve many substances. Some of its key applications include:
- Chemical Manufacturing: Used in the production of PVC, fertilizers, and dyes.
- Food Industry: Employed in the production of gelatin and other food additives.
- Metal Processing: Used for cleaning and treating metal surfaces.
- Water Treatment: Helps in the purification of water by adjusting pH levels.
Frequently Asked Questions
Q: Is HCl a strong acid?
A: Yes, HCl is a strong acid because it dissociates completely in water to form H⁺ and Cl⁻ ions.
Q: Why does HCl dissociate in water?
A: HCl dissociates in water due to its polar covalent nature, which allows it to interact with water molecules.
Q: Can HCl conduct electricity?
A: HCl can conduct electricity when dissolved in water because it dissociates into ions, but it does not conduct electricity in its pure form.
Conclusion
In conclusion, hydrochloric acid (HCl) is a polar covalent compound, not an ionic compound. Its bonding nature is determined by the electronegativity difference between hydrogen and chlorine, as well as its molecular structure. Understanding whether HCl is ionic or covalent is essential for predicting its behavior in chemical reactions and industrial applications.
We hope this article has provided you with a clear and comprehensive understanding of HCl's bonding nature. If you found this guide helpful, feel free to share it with others or leave a comment below. For more informative articles on chemistry and other topics, explore our website and stay updated with the latest insights!